Part VII: Other Issues
7.1 Management of Antibiotic Allergy
Background
Drug allergy (also known as hypersensitivity reactions) are adverse drug reactions (ADR) resulting from specific immune-mediated responses.
Most ADR are not allergy, but are often misdiagnosed and incorrectly labelled.
~7% of the Hong Kong population have reported drug ‘allergy’ labels in their medical records, of which the majority are to antibiotics (with one-third to β-lactams). [645]
However, up to 85% of these β-lactam ‘allergy’ labels are found to be incorrect after allergist evaluation. [646]
This pattern is consistent with different populations, evidenced by inter-population comparisons. [647,648]
Most antibiotic and penicillin ‘allergy’ labels in Hong Kong are created during adulthood, frequently mistaken for other non-allergic adverse drug reactions. [649]
In vivo tests (such as skin prick tests (SPT), intradermal tests (IDT) and patch tests (PT)) can be helpful in assisting with diagnosis but must be evaluated in the context of the individual’s clinical history. In vivo tests for certain antibiotics, e.g. piperacillin-tazobactam, have particularly poor predictive values and should be interpreted with caution. [650]
A major source of incorrect penicillin allergy mislabelling is due to inappropriate use (and interpretation) of penicillin skin tests still performed in Mainland China (>97% are false positive). [651]
In vitro tests, such as basophil activation tests, lymphocyte transformation tests, and enzyme-linked immunosorbent spot assays, are not routinely recommended and primarily performed within research institutes.
Mislabelled antibiotic allergy labels can lead to a myriad of adverse clinical outcomes: including increased morbidity and mortality, unnecessary use of second-line antibiotics, impaired health-related quality of life and development of MDRO. [652,653] It is, therefore, vital to correct mislabelled drug allergy labels and avoid unnecessary avoidance of mislabelled antibiotics.
Prevalence and potential harms of mislabelled antibiotic allergies are further exaggerated among particularly susceptible patient populations, including the elderly and immunocompromised. [654–656]
Assessment of patients with history of suspected antibiotic allergy
Although drug provocation test (DPT, also known as ‘drug challenges’) remains the gold standard, the most important diagnostic aid to antibiotic/drug allergy is the clinical history.
Many patients are mislabelled due to self-fear or incorrect concerns regarding suspected allergy: e.g. family history of allergy, non-immune mediated ADR (such as isolated gastrointestinal symptoms) or previously told skin testing positive (but no history of reaction).
A comprehensive history to ascertain all details pertaining the index reaction (i.e. the reaction which lead to the labelling of suspected antibiotic allergy) and subsequent reactions/tolerance of the same culprit drugs must be documented.
It is also useful to differentiate into immediate vs non-immediate/delayed (from clinical history) to guide subsequent investigations and management.
-
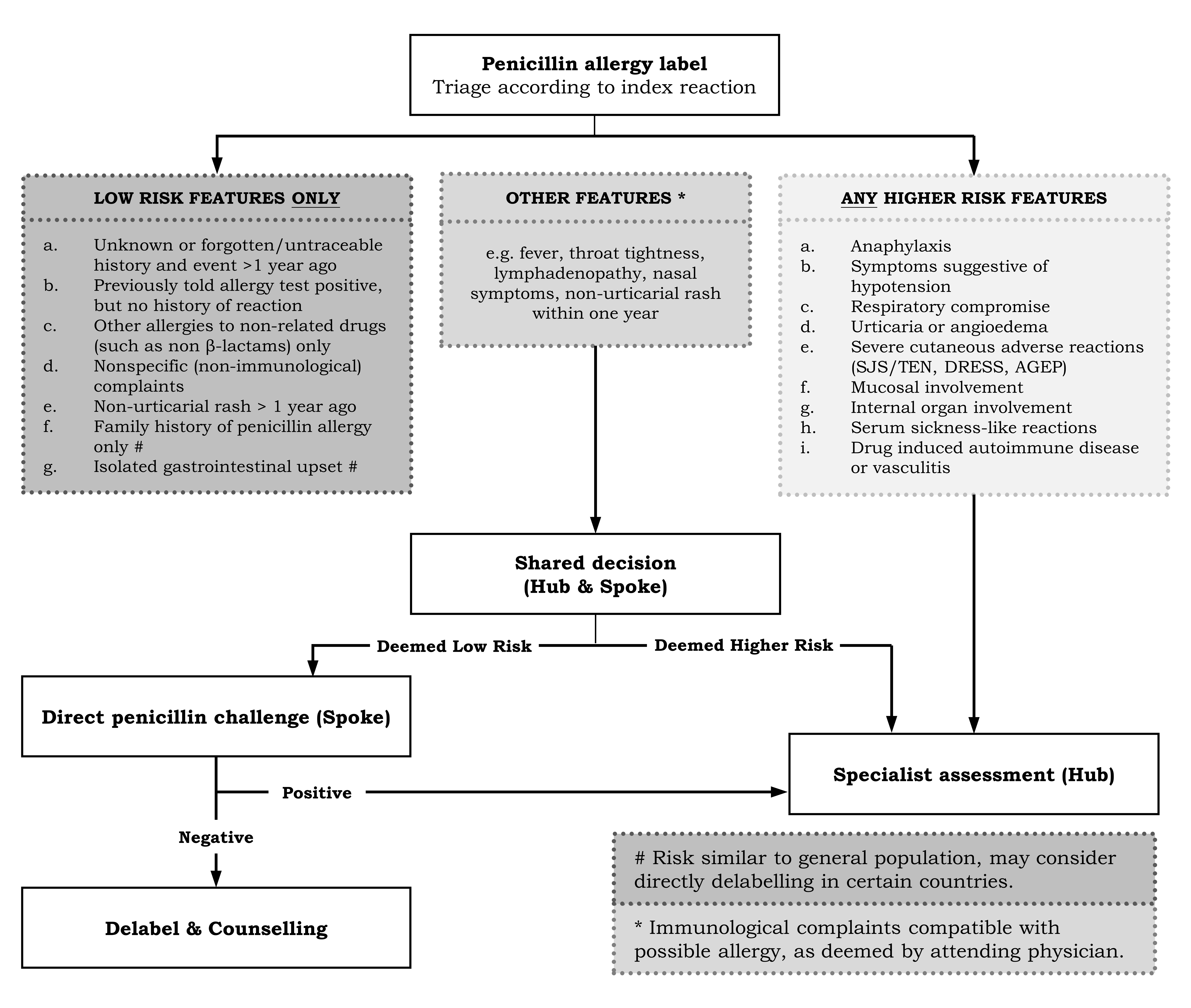
After history taking, the reaction should be stratified according to individual risk. For patients with suspected penicillin allergy, low- and high-risk features criteria used in the Asia Pacific are listed in Table 7.1. [657]
Most patients in Hong Kong with suspected penicillin allergy have ‘low-risk’ features only.
Low-risk patients are at low risk of genuine penicillin allergy and/or severe potential reactions.
For adult patients, the Hospital Authority currently employs a ‘Hub-and-Spoke’ approach [the Hong Kong Drug Allergy Delabelling Initiative (HK-DADI)]. HK-DADI currently accepts all referrals, from the public or private sectors, across the entire territory.
Designated ‘Spoke’ centres have been trained to perform allergy testing for patients with low-risk penicillin allergy (i.e. low-risk features only). The Hong Kong West Cluster (‘Hub’) provides advice, training and support for Spoke centres (Figure 7.1). [658]
All medical professionals (including clinicians, nurses, and pharmacists) need to be properly trained, and undergo periodic audits on practice, systems, and processes. Maintenance of competency through continuing medical education is also essential. [659]
If patients have low-risk features only, a direct DPT (without need for prior skin testing or in vitro test) may be considered at Spoke centres (Figure 7.1).
If patients have any high-risk features, then allergist review is necessary at the Hub.
If patients have other features (not specified as Low- or High-Risk), cases should be discussed with the Hub before proceeding.
Low-risk features |
High-risk features |
||
|---|---|---|---|
• |
Unknown or forgotten/untraceable history and event more than 1 year ago |
• |
Anaphylaxis |
• |
Previously told allergy test positive, but no history of reaction |
• |
Symptoms suggestive of hypotension |
• |
Other allergies to non-related drugs (such as non-β-lactams) only |
• |
Respiratory compromise |
• |
Non-specific (non-immunological) complaints |
• |
Urticaria or angioedema |
• |
Non-urticarial rash which occurred more than 1 year ago |
• |
Severe cutaneous adverse reactions (Stevens-Johnson syndrome/toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalised exanthematous pustulosis) |
• |
Family history of penicillin allergy only |
• |
Mucosal involvement |
• |
Isolated gastrointestinal upset |
• |
Internal organ involvement |
• |
Serum sickness-like reactions |
||
• |
Drug-induced autoimmune disease or vasculitis |
||
Drug provocation tests at Spoke centres
Negative DPTs can confidently rule out allergy and provide assurance in the process of delabelling.
Clinical assessments and DPT conducted by non-specialists have been demonstrated to be equally effective and safe for low-risk patients. [660,661]
Patients with the following conditions should not be evaluated for DPT at Spoke centres but instead have their evaluations deferred or be referred to the Hub:
Pregnancy;
Immunocompromised patient (or on systemic immunosuppression in the past 4 weeks);
Unable to withhold medications potentially interfering with DPT (e.g. antihistamines, tricyclic antidepressants);
Uncontrolled asthma, urticaria, or other diseases limiting the use of rescue medications.
DPT should be performed in an appropriate setting with resuscitation facilities readily accessible and under the supervision of trained personnel.
Medications potentially interfering with DPT (e.g. antihistamines) should be stopped for 7 days before DPT.
The index penicillin should be used for DPT (if known).
If the index penicillin is unknown, DPT should be performed with amoxicillin.
A graded approach to the maximum single dose (e.g. 3-step: 10%, 30%, 60%, or 2-step 10%, 90%) given at 30-minute intervals is recommended.
Patient should be observed at least 1 hour after the final dose of DPT, with clear instructions on what to do if symptoms develop after leaving.
An immediate-type hypersensitivity to the DPT agent is confidently excluded if there is no reaction after >1 hour after completion of DPT.
Patients should be contacted at least 72 hours later to ensure there were no non-immediate type manifestations.
A DPT is considered negative if there is no definite reaction after at least 72 hours after the completion of the DPT.
Patients with reported reactions after DPT should be called back for review at Spoke centres and treated, if and as necessary.
Patients with equivocal reactions can be offered repeat DPT or referred to the Hub.
Inaccurate penicillin allergy labels should be delabelled following a negative DPT with proper patient counselling and written documentation (such as a drug allergy notification letter).
Skin testing for suspected penicillin allergy
Skin testing should only be performed when there is clinical suspicion of possible drug allergy.
SPT and IDT (immediate reading) are useful for suspected immediate-type reactions.
PT and IDT (delayed reading) are useful for suspected non-immediate-type reactions.
If indicated, skin testing should be performed at least 8 weeks after (and as soon as possible) following history of suspected allergic reaction.
Antihistamines and tricyclic antidepressants should be withheld at least 1 week prior to skin testing.
Positive (histamine) and negative controls must be used for SPT and IDT.
Regarding drug dilutions and reagents for SPT and IDT:
SPT followed by IDT at the highest non-irritating concentration should be performed.
All SPT should be accompanied by a positive and negative control.
All IDT should be accompanied by a negative control.
SPT and IDT should be performed using recommended concentrations of benzylpenicilloyl-poly-L-lysine (5×10-5 mmol/L), minor determinant mixture (2×10-5 mmol/L), benzylpenicillin (10,000 units/mL), amoxicillin (20 mg/mL) and culprit drug (if known).
- Regarding interpretation of SPT and IDT:
SPT is considered positive if a wheal size diameter at least 3 mm larger than negative control, with surrounding erythema.
IDT is considered positive if diameter of the wheal is at least 3 mm greater than the initial wheal, with surrounding erythema.
Delayed IDT readings at 48–72 hours may be considered if a non-immediate type reaction is suspected.
Patients with positive SPT or IDT results should be referred for specialist review.
PT can be performed by diluting the drugs with petrolatum or aqueous (10%), prepared and interpreted as per the International Contact Dermatitis Research Group.
Following skin testing, DPT is the gold standard for diagnosis and remains necessary to prove tolerance.
DPT should generally be performed when there is a low pre-test probability (and following negative skin testing, if performed) as described above.
Managing suspected allergic reactions to antibiotics
The first step in identifying allergic reactions is to classify them into immediate (Type I) or non-immediate (Type IV) types.
Type II (e.g. drug-induced cytopaenias, hepatitis, nephritis) and Type III (serum sickness, vasculitis, drug fever) reactions are much less common and not amenable to traditional drug allergy testing.
Immediate-type reactions are classically IgE-mediated, and typically occur within 1 hour if there has been prior exposure.
However, immediate-type reactions can also occur after several days if it is during the first treatment course.
Manifestations of immediate-type reactions are typically related to mast-cell degranulation, including urticaria (hives and/or angioedema), bronchospasm, abdominal pain, diarrhoea or anaphylactic shock.
Immediate-type reactions should be treated initially according to the local anaphylaxis protocols, and if there are any signs of systemic involvement, intramuscular adrenaline should be administered as soon as possible. Anti-histamines and systemic corticosteroids only serve as an adjunct. After the patient has stabilised, serum should be collected and saved for acute tryptase levels. The sample can be stored as clotted blood at 4°C, and an acute sample should be saved preferably at 30 minutes to 4 hours after the event. In addition, a baseline sample should be taken after >24 hours following the event.
If a patient has a confirmed immediate-type hypersensitivity, desensitisation may be an option, but only after consultation with an allergist and if there are no alternative medications available.
Non-immediate (delayed)-type reactions are classically T-cell-mediated, and typically occur >1 hour (up to days/weeks) administration and lesions can usually last for days to weeks.
Cutaneous manifestations are not urticarial in nature, and can include maculopapular or morbilliform rashes, erythema multiforme, fixed drug eruptions, contact dermatitis.
The most severe non-immediate reactions are the Severe Cutaneous Adverse Reactions (SCAR), which Drug reaction with eosinophilia & systemic symptoms (DRESS) syndrome, acute generalised exanthematous pustulosis (AGEP), Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Mortality for SCAR can reach up to ~30%.
Some SCAR may occur weeks to months (especially DRESS syndrome and SJS/TEN) following drug exposure and may be missed without high index of suspicion.
Most reactions resolve after withdrawal of causative agent, but severe cutaneous reactions which may warrant steroids or immunomodulation.
SCAR usually contraindicates further re-exposure to suspected culprit drugs.
Desensitisation for non-immediate reactions are seldom possible and ‘treating through’ not routinely recommended.
Documentation of newly suspected drug allergy
All documentation should be clearly recorded in medical records and provided to the patient, (either in written form or drug alert bracelet/jewellery), whenever feasible.
Drug allergy (immune-mediated) should be clearly differentiated from and documented separately with non-immune mediated ADR, whenever possible.
Documentation of all suspected or confirmed drug allergies should include, at a minimum, the names of suspected culprits (preferably with both generic and proprietary names, if known), dates and manifestations of all reactions, and timing of reactions (immediate, within 1 hour, vs. non-immediate, >1 hour).
If available, the reason for drug prescription, method of administration, necessity for treatment, or requirement for hospitalisation due to suspected drug reactions should also be included.
If the patient has received medications belonging to the same labelled drug class(es) AFTER their index reaction, subsequent reactions or tolerance to these specific agents should also be recorded (including name of drug and date of reaction or tolerance).
Relevant drug allergy investigations (especially drug provocation or tolerance tests) with details of drug names and dates should also be documented.
Choosing alternative antibiotics for patients with suspected penicillin allergy
Since most penicillin ‘allergy’ labels are incorrect, the most important step is to confirm the index allergy label, as described above.
If there is a dubious allergy history, a graded drug provocation test may be a suitable strategy for low-risk individuals, with the patient’s consent.
For patients with a confirmed penicillin allergy, there is no contraindication for carbapenem use, although consulting with an allergist to test for suitable alternatives may be considered. [662]
Cross-reactivity between penicillin and cephalosporins is generally low, and is usually due to recognition of similar side chains.
Therefore, if cephalosporins are the desired treatment (and no other alternatives are available), it is advisable to select a cephalosporin with dissimilar side-chains from that of the index offending penicillin (Table 7.2). After consulting with an allergist, skin testing or in vitro testing can be performed to test selected cephalosporins, if possible.
If there are no alternative antibiotics available, and penicillins are the desired treatment, desensitisation may be possible for patients with a history of immediate-type reactions.
Although the need for desensitisation is low due to the recognition of incorrect antibiotic allergy labels and the availability of more antibiotic alternatives, the decision to proceed with desensitisation should be a shared decision made by allergists and patients, balancing individualised risk versus benefit.
|
Amoxicillin |
Ampicillin |
Cefaclor |
Cefadroxil |
Cefepime |
Cefoperazone |
Cefotaxime |
Cefoxitin |
Cefpodoxime |
Ceftazidime |
Ceftibuten |
Ceftriaxone |
Cefuroxime |
Cephalexin |
Cephaloridine |
Cephalothin |
Cephradine |
Penicillin G |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Amoxicillin |
6 |
6/7 |
6/7 |
6/7 |
6/7 |
|||||||||||||
Ampicillin |
6 |
6/7 |
6/7 |
6/7 |
6/7 |
|||||||||||||
Cefaclor |
6/7 |
6/7 |
7 |
7 |
7 |
|||||||||||||
Cefadroxil |
6/7 |
6/7 |
7 |
3,7 |
3,7 |
|||||||||||||
Cefepime |
7 |
7 |
7 |
|||||||||||||||
Cefoperazone |
||||||||||||||||||
Cefotaxime |
7 |
7 |
7 |
3 |
||||||||||||||
Cefoxitin |
3 |
7 |
7 |
6/7 |
||||||||||||||
Cefpodoxime |
7 |
7 |
7 |
|||||||||||||||
Ceftazidime |
||||||||||||||||||
Ceftibuten |
||||||||||||||||||
Ceftriaxone |
7 |
7 |
7 |
|||||||||||||||
Cefuroxime |
3 |
|||||||||||||||||
Cephalexin |
6/7 |
6/7 |
7 |
3,7 |
3,7 |
|||||||||||||
Cephaloridine |
7 |
7 |
6/7 |
|||||||||||||||
Cephalothin |
3 |
7 |
7 |
6/7 |
||||||||||||||
Cephradine |
6/7 |
6/7 |
7 |
3,7 |
3,7 |
|||||||||||||
Penicillin G |
6/7 |
6/7 |
6/7 |
|||||||||||||||
Numbers denote position of side chains: 3, similarity at the cephalosporin 3—position side chain; 7, similarity at the cephalosporin 7—position side chain; 6/7, similarity at the penicillin 6-position side chain and the cephalosporin 7-position side chain. | ||||||||||||||||||
Each number in the matrix indicates side-chain similarity between two drugs. Cross-allergenicity is expected between each similar pair. For example, a patient allergic to amoxicillin would very likely manifest an allergic reaction to ampicillin, cefadroxil, cefaclor, cephalexin and cephradine. However, the patient would not be expected to exhibit an allergic response to cefepime, cefoperazone, cefotaxime, etc., unless he/she was also allergic to another cephalosporin or penicillin with a similar side chain to the reference drug. | ||||||||||||||||||
Reference: [663]