Home > Chapters
Chapters
Part IV: Recommendation for the empirical therapy of common infections
4.3 Management of community-acquired pneumonia (CAP)
4.3.1 General considerations and principles
- A number of guidelines on the management of CAP were released or updated recently. While these guidelines were drawn on the basis of the same set of literature, patient stratification and specific suggestions still vary quite a bit (326,330–332).
- Newer studies (375–376) continue to support the notion stated in guidelines that S. pneumoniae is one of the most common pathogens identified in CAP. Hence, the choice of agents for empirical therapy should consider the regional data on prevalence and risk factors for drug-resistant S. pneumoniae (DRSP).
- Appropriate antimicrobial therapy should be initiated as soon as possible (333,377–378).
- Factors to be considered in choosing empirical therapy for CAP:
- Place of therapy (outpatient, inpatient ward, or ICU).
- Role of atypical pathogens (e.g. Chlamydophila pneumoniae, Mycoplasma pneumoniae and Legionella spp.) is increasingly being recognised. Coverage for atypical pathogens should always be given for hospitalised patients with moderate to severe disease, although it is considered optional for non-hospitalised patients with low-severity CAP (326,330).
- Presence of modifying factors including risk factors for DRSP (e.g. age >65 years, ß-lactam therapy within past 3 months, alcoholism, multiple medical comorbidities, exposure to a child in a day care centre), enteric Gram-negatives (residence in a nursing home, underlying cardiopulmonary disease, multiple medical comorbidities, recent antibiotic therapy), and P. aeruginosa (e.g. bronchiectasis).
- Emerging resistance patterns among the major pathogens. In Asia, including HK, high prevalence of macrolide resistance has been reported among Mycoplasma pneumoniae strains in recent years (113–115,118,379–380).
- Emerging pathogens including those of regional significance such as CA-MRSA (association with necrotising pneumonia and influenza virus coinfection), Klebsiella pneumoniae (association with disseminated infection, liver abscess and diabetes mellitus) and Burkholderia pseudomallei (occur in melioidosis endemic area during rainy season) (381–382).
- Several antibiotics active against P. aeruginosa, including cefepime, imipenem, meropenem and piperacillin-tazobactam are generally active against DRSP. They can be used for patients having specific risk factors for P. aeruginosa.
- If a macrolide is relied upon for coverage of H. influenzae, the newer macrolides (e.g. clarithromycin or azithromycin) should be used instead of erythromycin.
- For most patients, appropriately chosen initial antibiotic therapy should not be changed in the first 72 hours, unless there is marked clinical deterioration.
- Most patients with CAP will have an adequate clinical response within 72 hours. After the patient has met appropriate criteria, switch from I.V. to P.O. therapy can be made.
4.3.2 Management of community-acquired pneumonia (CAP) in the era of pneumococcal resistance: conclusions from the CDC working group
- Comparative studies of adults and children have reported that pneumonia due to penicillin-nonsusceptible pneumococci (most had MIC>0.1–1 μg/mL) does not influence the outcome of pneumonia treatment (383–384). At higher level of resistance (penicillin MIC 2–4 μg/mL), recent evidence suggests that risk of mortality or suppurative complications were increased (385–386). In one study (387), the observed increase in mortality was confined to patients with pneumococcal isolates with penicillin MIC of ≥4 μg/mL.
- Since 2012, different breakpoints have been used for interpretation of penicillin susceptibility according to the site of infections and route of drug administration (388–389).
Table 4.3 Interpretation of penicillin susceptibility for S. pneumoniaeSyndrome, route of administration and agent Penicillin or amoxicillin MIC (μg/mL) Susceptible Intermediate Resistant Meningitis, Parenteral penicillin ≤ 0.06 - ≥ 0.12 Non-meningitis, Parenteral penicillin ≤ 2 4 ≥ 8 Non-meningitis, Oral (high dose) amoxicillin or amoxicillin-clavulanic acid ≤ 2 4 ≥ 8 Oral penicillin V ≤ 0.06 0.12–1 ≥ 0.12
By modifying the breakpoints, it is hope that there will be decreased use of broad-spectrum antimicrobial therapy in favour of more narrow-spectrum therapy. Patients with pneumococcal pneumonia caused by strains with penicillin MIC ≤1 μg/mL can be treated appropriately with optimal dosage of I.V. penicillin and several other P.O./I.V. ß-lactams. Comparative anti-pneumococcal activities of commonly used ß-lactams are shown in Table 4.4. - Vancomycin is not routinely indicated for treatment of CAP or for pneumonia caused by DRSP.
- The CDC working group does not advocate the use of newer fluoroquinolones for first line treatment of CAP. The reasons are:
- Most penicillin-nonsusceptible S. pneumoniae pneumonia can be appropriately treated with a ß-lactam with good anti-pneumococcal activity at optimal dosage.
- Concerns that resistance among pneumococci will rapidly emerge after widespread use of this class of antibiotics.
- Their activity against pneumococci with high level penicillin resistance (MIC ≥4 μg/mL) makes it important that they should be reserved for selected patients with CAP.
- Indications for use of fluoroquinolones in CAP
- Adults for whom one of the first line regimen has already failed.
- Allergic to alternative agents.
- Documented infection due to pneumococci with high level penicillin resistance (penicillin MIC ≥4 μg/mL).
4.3.3 Regional considerations for S. pneumoniae
- In HK, reduced susceptibility to penicillin (Figure 4.2) and resistance to macrolides were high in both hospital (328,390–393) and community settings (394–397). Recent evidence suggests increase in carriage of certain serotypes (such as 15) after introduction of childhood vaccination by pneumococcal conjugate vaccine-13 (392–393,397), although the significance of this phenomenon remains uncertain at this stage.
- Erythromycin resistant isolates are also resistant to the newer macrolides/azalides such as clarithromycin and azithromycin (398). In 2012–2016, the age group-specific rates of macrolide resistance among 775 invasive pneumococcal isolates were as follows: 76% in <5 years, 92% in 5–17 years, 74% in 18–64 years and 75% in ≥65 years. Accordingly, macrolides should not be used as sole therapy for empirical treatment of presumed pneumococcal infection.
- Globally, resistance to fluoroquinolones among the pneumococci is low (<1–2%). HK is one of the rare exceptions in which fluoroquinolone resistance (levofloxacin MIC ≥8 μg/mL) is emerging among the S. pneumoniae, especially among respiratory isolates from elderly patients with chronic lung diseases (390). One regional study found an association between levofloxacin resistance and mortality in adult patients with invasive pneumococcal disease (399).
- In view of the above, adherence to the CDC guidelines on the use of the fluoroquinolones seems appropriate. Moreover, tuberculosis (TB) is prevalent in HK and was reported to account for ~10% of CAP in the
elderly. Excess use of fluoroquinolones in CAP may lead to: (1) delay in
diagnosis of TB; (2) increased fluoroquinolone resistance among
Mycobacterium tuberculosis (400–401). Hence, this class of agents is not
recommended as first line (or routine) therapy in HK for CAP. In this
regard, extra care need to be exercised in using fluoroquinolones in
patients with risk factors for fluoroquinolone-resistant S. pneumoniae (402–403) :
- Presence of chronic obstructive pulmonary disease;
- Underlying cerebrovascular disease;
- Residence in old age home;
- Past exposure to fluoroquinolones; and
- Healthcare-associated/nosocomial pneumococcal infection.
- Ciprofloxacin and ofloxacin should not be used to treat pneumococcal infection. Use of a suboptimal dose of fluoroquinolone should be avoided (e.g. the dose/frequency approved by FDA for levofloxacin in CAP is 500 mg/day). Use of <500 mg and in divided doses should be avoided as these have been showed to be associated with the emergence of fluoroquinolone-resistant S. pneumoniae (329). If a respiratory fluoroquinolone is indicated, there is evidence to suggest that the more potent ones (e.g. moxifloxacin) are less likely to lead to development of resistance.
- Penicillin G (I.V.) or ampicillin (P.O./I.V.) or amoxicillin (P.O./I.V.) are generally viewed as the ß-lactam drugs of choice for treating infections with penicillin-susceptible and penicillin-intermediate strains of S. pneumoniae. The following ß-lactams are not recommended because of poor intrinsic activities against S. pneumoniae: penicillin V, all first generation cephalosporins, cefaclor, cefixime, ceftibuten, and loracarbef.
- Lung infections involving strains with intermediate susceptibility to penicillin (MIC 0.1–1 μg/mL) may be treated with I.V. penicillin G or P.O. amoxicillin (high dose).
- Penicillins combined with ß-lactamase inhibitors (ampicillin-sulbactam, amoxicillin-clavulanate, piperacillin-tazobactam) are active against ß-lactamase-producing organisms including H. influenzae, M. catarrhalis, and methicillin-sensitive S. aureus. Except in patients with mixed infection, these drugs offer no advantage over penicillin G or amoxicillin for the treatment of S. pneumoniae pneumonia, including those due to penicillin-resistant strains because ß-lactamase is not produced by S. pneumoniae. The MIC of ampicillin, amoxicillin, piperacillin for most local strains were similar to that of penicillin. However, the MIC of ticarcillin is increased disproportionately among penicillin non-susceptible strains.
Figure 4.2 Susceptibility of 775 invasive pneumococcal isolates to penicillin and cefotaxime according to patient age groups, 2012–2016, HK
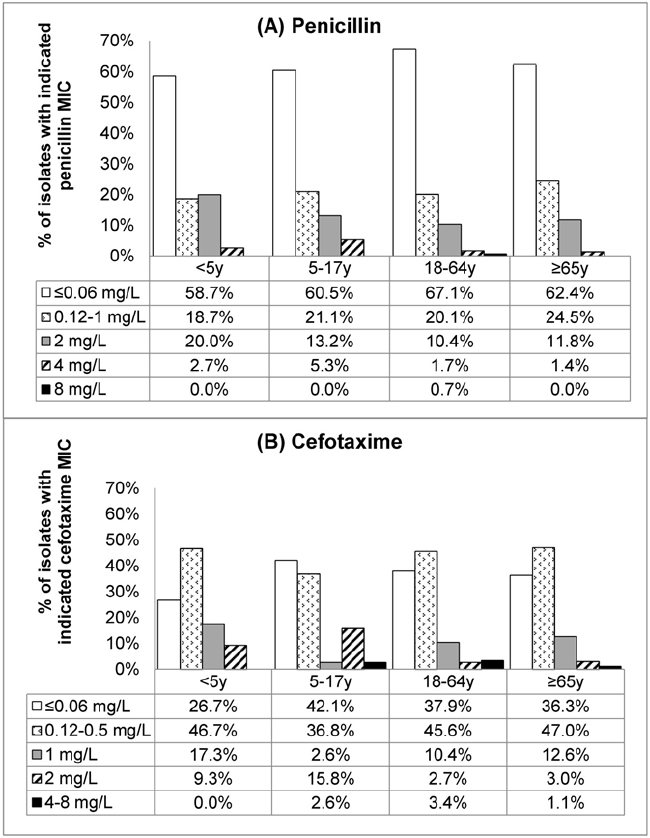
Table 4.4 Comparative activities of commonly used ß-lactams against S. pneumoniae with different levels of penicillin susceptibility
| Agent | Penicillin MIC | |||
|---|---|---|---|---|
| ≤0.06 μg/mL | 0.12−1 μg/mL | 2 μg/mL | ≥4 μg/mL | |
| Penicillin V | +++ | + | - | - |
| Penicillin G | +++ | +++ | ++ | ± |
| Ampicillin P.O. | +++ | ++ | ± | - |
| Ampicillin I.V. | +++ | +++ | ++ | ± |
| Amoxicillin P.O. | +++ | ++ | + | - |
| Piperacillin | +++ | ++ | + | - |
| Ticarcillin | ++ | + | - | - |
| Cefotaxime | +++ | +++ | ++ | ± |
| Ceftriaxone | +++ | +++ | ++ | ± |
| Cefepime | +++ | ++ | + | ± |
| Cefuroxime I.V. | +++ | ++ | + | - |
| Cefuroxime P.O. | +++ | ++ | ± | - |
| Cefpodoxime | +++ | ++ | - | - |
| Ceftazidime | +++ | + | - | - |
| Cefaclor | +++ | - | - | - |
| Cefixime/ceftibuten | +++ | - | - | - |
| Imipenem/meropenem | +++ | +++ | + | - |
Penicillin MIC interpretation criteria (μg/mL) for I.V. penicillin G: meningitis ≤0.06 sensitive, ≥0.12 resistant; nonmeningitis ≤2 sensitive, 4 intermediate and ≥8 resistant.
Approximate in vitro activity was indicated by: - inactive, + weak activity, ++ good activity, +++ excellent activity, ± variable or dose-dependent