Part IV: Recommendation for the Empirical Therapy of Common Infections
4.2 Management of Community-Acquired Pneumonia (CAP)
General Considerations and Principles
-
A number of guidelines on the management of CAP were released or updated recently. While these guidelines were drawn on the basis of the same set of literature, patient stratification and specific suggestions still vary. [472–475]
-
The implementation of rapid, multiplex PCR assays may enhance the management of severe community-acquired pneumonia when non-standard antibiotics are utilised. However, performing multiplex PCR on sputum specimens may result in a large number of false-positive results from the oropharyngeal flora. [475,482,483]
-
S. pneumoniae remains one of the most common pathogens identified in CAP [484–487] as stated in the guidelines. Hence, the choice of agents for empirical therapy should consider the regional data on prevalence and risk factors for drug-resistant S. pneumoniae (DRSP).
-
Factors to be considered in choosing empirical therapy for CAP:
Place of therapy (outpatient, inpatient ward, or ICU).
Role of atypical pathogens (e.g. Chlamydia pneumoniae, Mycoplasma pneumoniae and Legionella spp.) is increasingly being recognised. Coverage for atypical pathogens should always be given for hospitalised patients with moderate to severe disease, although it is considered optional for non-hospitalised patients with low-severity CAP. [472,473]
Presence of modifying factors including risk factors for DRSP (e.g. age >65 years, β-lactam therapy within past 3 months, alcoholism, multiple medical comorbidities, exposure to a child in a day care centre), enteric Gram-negatives (residence in a nursing home, underlying cardiopulmonary disease, multiple medical comorbidities, recent antibiotic therapy), and P. aeruginosa (e.g. bronchiectasis).
Emerging resistance patterns among the major pathogens. In Asia, including Hong Kong, high prevalence of macrolide resistance has been reported among Mycoplasma pneumoniae strains in recent years. [26,125,126,488–491]
Burkholderia pseudomallei is endemic in Hong Kong. An increase in cases of Melioidosis may be expected during summer, when heavy rainfalls and tropical cyclones can expose the bacteria previously buried in soil to the ground surface. Airborne transmission was postulated to be involved in a local outbreak in 2022. [492–496]
-
Certain antibiotics active against P. aeruginosa including cefepime and piperacillin-tazobactam are generally active against DRSP. They can be used for patients having specific risk factors for P. aeruginosa.
-
For most patients, appropriately-chosen initial antibiotic therapy should not be changed in the first 72 hours, unless there is marked clinical deterioration.
-
Most patients with CAP will have an adequate clinical response within 72 hours. After the patient has met appropriate criteria, switch from I.V. to P.O. therapy can be made.
Management of Community-Acquired Pneumonia in the Era of Pneumococcal Resistance
-
Comparative studies of adults and children have reported that pneumonia due to penicillin-nonsusceptible pneumococci (most had MIC >0.1–1 microgram/mL) does not influence the outcome of pneumonia treatment. [497,498] At higher level of resistance (penicillin MIC 2–4 microgram/mL), recent evidence suggests that risk of mortality or suppurative complications were increased. [499,500] In one study, [501] the observed increase in mortality was confined to patients with pneumococcal isolates with penicillin MIC of ≥4 microgram/mL.
-
Since 2012, different breakpoints have been used for interpretation of penicillin susceptibility according to the site of infections and route of drug administration. [60,502]
Syndrome, route of administration and agent |
Penicillin or amoxicillin MIC (microgram/mL) |
||
|---|---|---|---|
Susceptible |
Intermediate |
Resistant |
|
Meningitis, Parenteral penicillin |
≤0.06 |
- |
≥0.12 |
Non-meningitis, Parenteral penicillin |
≤2 |
4 |
≥8 |
Non-meningitis, Oral (high dose) amoxicillin or amoxicillin-clavulanate |
≤2 |
4 |
≥8 |
Oral penicillin V |
≤0.06 |
0.12–1 |
≥2 |
-
By modifying the breakpoints, it is hoped that there will be decreased use of broad-spectrum antimicrobial therapy in favour of more narrow-spectrum therapy. Patients with pneumococcal pneumonia caused by strains with penicillin MIC ≤1 microgram/mL can be treated appropriately with optimal dosage of I.V. penicillin and several other P.O./I.V. β-lactams. Comparative anti-pneumococcal activities of commonly used β-lactams are shown in Table 4.2.
-
Vancomycin is not routinely indicated for treatment of CAP or for pneumonia caused by DRSP.
-
Newer fluoroquinolones are not recommended as first-line treatment of CAP. [503] The reasons are:
Most penicillin-nonsusceptible S. pneumoniae pneumonia can be appropriately treated with a β-lactam with good anti-pneumococcal activity at optimal dosage. [504,505]
Concerns that resistance among pneumococci will rapidly emerge after widespread use of this class of antibiotics.
Their activity against pneumococci with high level penicillin resistance (MIC ≥4 microgram/mL) makes it important that they be reserved for selected patients with CAP.
Agent |
Penicillin MIC |
|||
|---|---|---|---|---|
≤0.06 microgram/mL |
0.12–1 microgram/mL |
2 microgram/mL |
≥4 microgram/mL |
|
Penicillin V |
+++ |
+ |
− |
− |
Penicillin G |
+++ |
+++ |
++ |
± |
Ampicillin P.O. |
+++ |
++ |
± |
− |
Ampicillin I.V. |
+++ |
+++ |
++ |
± |
Amoxicillin P.O. |
+++ |
++ |
+ |
− |
Piperacillin |
+++ |
++ |
+ |
− |
Ticarcillin |
++ |
+ |
− |
− |
Cefotaxime |
+++ |
+++ |
++ |
± |
Ceftriaxone |
+++ |
+++ |
++ |
± |
Cefepime |
+++ |
++ |
+ |
± |
Cefuroxime I.V. |
+++ |
++ |
+ |
− |
Cefuroxime P.O. |
+++ |
++ |
± |
− |
Cefpodoxime |
+++ |
++ |
− |
− |
Ceftazidime |
+++ |
+ |
− |
− |
Cefaclor |
+++ |
− |
− |
− |
Cefixime/ceftibuten |
+++ |
− |
− |
− |
Imipenem/meropenem |
+++ |
+++ |
+ |
− |
Regional Considerations for Streptococcus pneumoniae
-
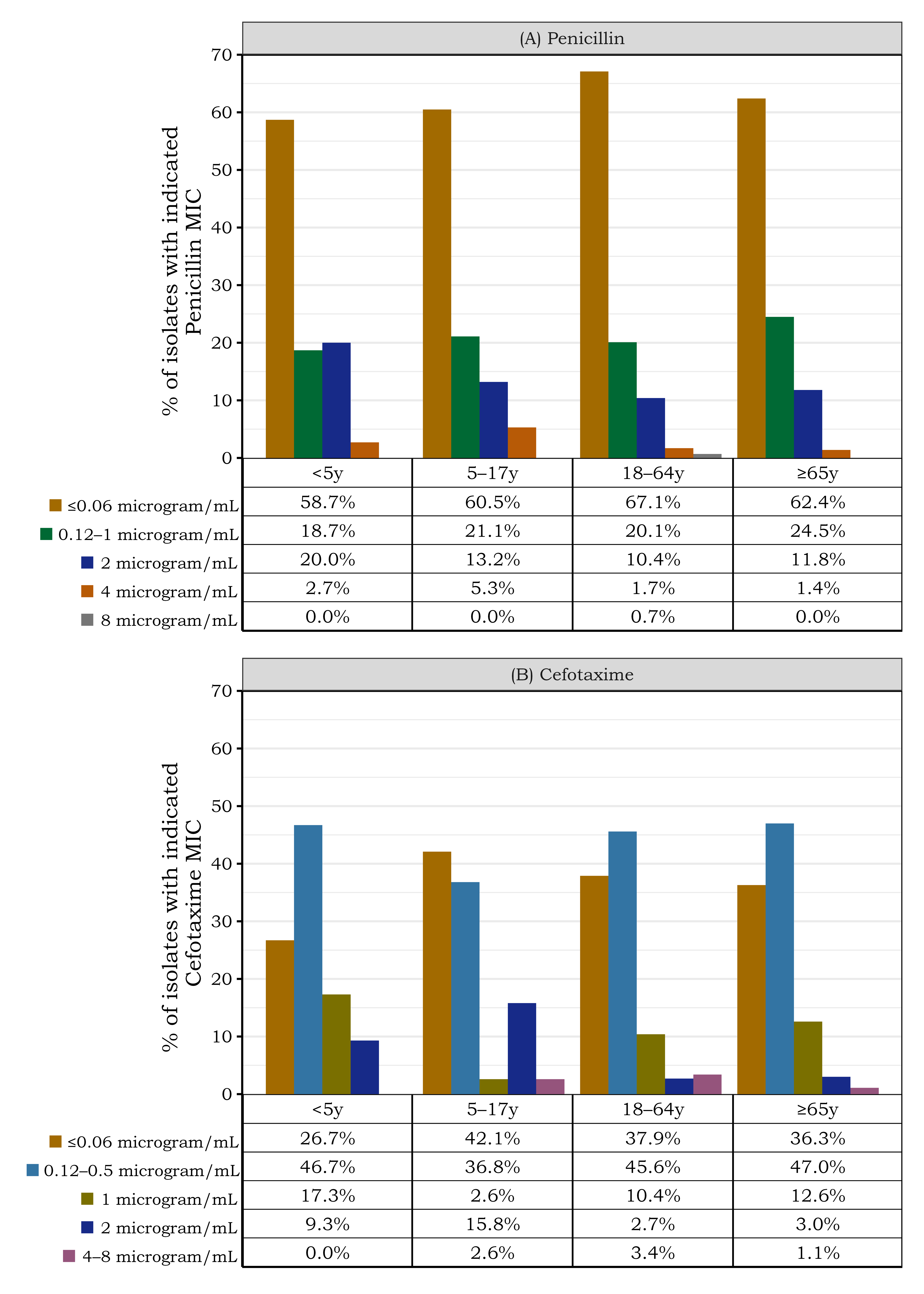
In Hong Kong, reduced susceptibility to penicillin (Figure 4.1) and resistance to macrolides were high in both hospital [506–510] and community settings. [511–515] Recent evidence suggests an increase in carriage of certain serotypes (such as 15) after introduction of childhood vaccination by pneumococcal conjugate vaccine-13, [507,508,512] although the significance of this phenomenon remains uncertain at this stage. [5,6,506–514,516–519]
-
Erythromycin-resistant isolates are also resistant to the newer macrolides/azalides such as clarithromycin and azithromycin. [520] In 2012–2016, the age group-specific rates of macrolide resistance among 775 invasive pneumococcal isolates were as follows: 76% in <5 years, 92% in 5–17 years, 74% in 18–64 years and 75% in ≥65 years. Accordingly, macrolides should not be used as sole therapy for empirical treatment of presumed pneumococcal infection.
-
In Hong Kong, fluoroquinolone resistance (levofloxacin MIC ≥8 microgram/mL) is emerging among S. pneumoniae, especially among respiratory isolates from elderly patients with chronic lung diseases. [507] Other risk factors for fluoroquinolone-resistant S. pneumoniae include residence in old age home or nosocomial pneumococcal infections. [519,521]
-
Moreover, tuberculosis (TB) was reported to account for ~10% of CAP in the elderly. [522] Excess use of fluoroquinolones in CAP may lead to: (1) delay in diagnosis of TB; (2) increased fluoroquinolone resistance among Mycobacterium tuberculosis. [523–525]
-
Ciprofloxacin and ofloxacin should not be used to treat pneumococcal infection. Use of a suboptimal dose of levofloxacin (e.g. <500 mg daily or in divided dose), which has been shown to be associated with the emergence of fluoroquinolone-resistant S. pneumoniae, should be avoided. [477]
-
The following β-lactams are not recommended because of poor intrinsic activities against S. pneumoniae: penicillin V, all first-generation cephalosporins, cefaclor, cefixime, ceftibuten, and loracarbef. [503]
-
Penicillins combined with β-lactamase inhibitors (ampicillin-sulbactam, amoxicillin-clavulanate, piperacillin-tazobactam) offer no advantage over penicillin G or amoxicillin for the treatment of pure pneumococcal pneumonia, including penicillin-resistant strains because S. pneumoniae does not produce β-lactamase. The MIC of ampicillin, amoxicillin, piperacillin for most local strains were similar to that of penicillin. However, the MIC of ticarcillin is increased disproportionately among penicillin non-susceptible strains.